CSO Corner Volume 4: Creating a Disease Concept Model
Hello fellow members of our KS family!
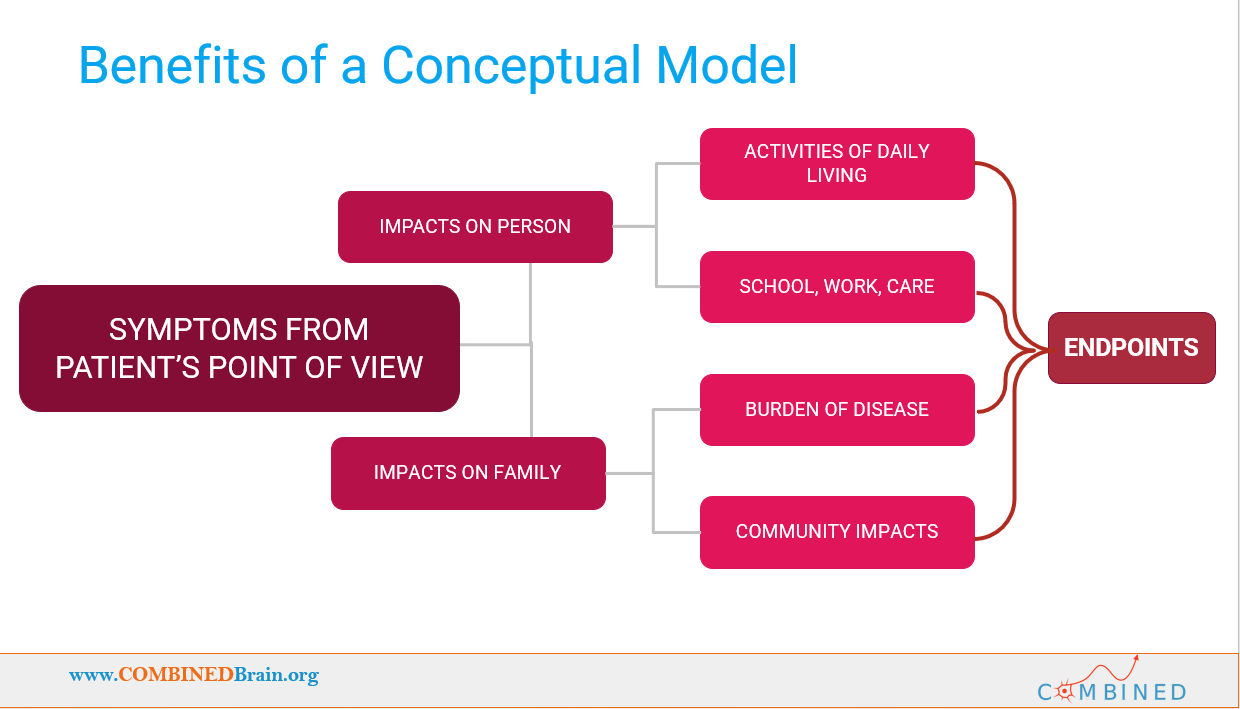
For this newsletter, I’d like to talk about disease concept models, and why IDefine is sponsoring a project at Boston University and Boston Children’s Hospital to create a disease concept model (DCM) for Kleefstra Syndrome (KS).
When you think about how your loved one has experienced KS, what comes to mind? What have been the challenges in daily living? In health? In interactions with others? What have the challenges been for you as a caregiver? How have those experiences changed over time as they have grown up? I’m sure many things come to mind. At its very heart, a DCM is a rigorous way to capture this “lived experience” of the disease.

So how will we create a DCM for KS? By a similar exercise to the paragraph above: by asking people! To create a DCM, you interview people with knowledge of the condition (parents, caregivers, clinicians, therapists, etc.), based on an interview guide. To develop the interview guide, you first leverage the substantial information already available about KS in the literature, to ensure that you cover all the known areas of the condition thoroughly. Importantly however, you also build a guide that provides plenty of open-ended questions that provide the opportunity for interviewees to add new information about the disorder (for example, less common manifestations that haven’t been reported yet). You also try to sample as diverse a group of people as possible, to get the most complete set of perspectives possible.
Once you have all the answers to your questions, you then “code” them into a structured classification system that makes the data tractable and comparable between interviews. Over time, with each interview, you build a more and more complete understanding of the condition, which eventually “saturates.” That is, additional interviews don’t add additional information.
This concept of saturation is important: Although we all probably feel like we have a really good handle on KS (given the front-row seat), each of us has really only experienced a subset of the full-lived experience. Different families and patients will have different experiences and different perspectives. With a DCM, the goal is to try to capture it all.
Once you assemble all the data, you perform additional analysis and then write up the findings in the form of a scientific paper. Your DCM can then be shared with the public, so that everyone who wants to learn more about the disorder can do so. For a great example of the end product, see the DCM paper that was recently published for STXBP1.
Another important aspect of DCMs is that they are not meant to be put on a shelf, they are meant to be actively used! By defining the challenges presented by the disorder, a DCM provides a roadmap to what would constitute “improvement” in the disorder. This provides vital guidance to clinicians who may have little direct experience with the disorder, and to those designing clinical trials, when selecting endpoints for those trials. Thus, by building a DCM we put our community in a better position to receive improved care, and to be more prepared for a potential future clinical trial.
In fact, on that last point, the FDA now specifically expects this type of input to be considered by designers of clinical trials. This is all part of the FDA move towards “patient-focused drug development.” They really do want to know what we as a community would consider to be improvement, and they give a great deal of weight to that input when evaluating the results of clinical trials.
One more thing: as you might have guessed, Boston University and Boston Children’s Hospital won’t be able to do this DCM study without members of our KS family, just like you! The study will recruit for interviewees later this year, and I hope you will consider joining the study and providing your perspective.
Until next time,
Eric Scheeff, PhD
IDefine Chief Scientific Officer

