CSO Corner Volume 5: ASOs
Hello fellow members of our KS family!
For this newsletter, I’d like to talk about antisense oligonucleotides, otherwise known as the technology that is such a mouthful to pronounce that everyone just calls them “ASOs.” This technology has generated a lot of excitement, and led to FDA approved therapies for several disorders, with many more in clinical trials and pre-clinical development.
To really get into how ASOs work, we will have to delve a little bit into molecular biology, and it will be useful to have an analogy. So, I’d like to use the manufacturing of cars as that analogy. Cars (and other motor vehicles, for that matter) do a tremendous about of work in our society and fulfill many different roles. They also come in many forms that lead to many functions. And that makes them something like proteins in cells.
Proteins do most of the work in cells, and there are thousands of distinct protein types in human cells, each with their own roles. There are protein types that build structures, allow the cell to move, repair damage, remove trash, regulate cellular activity, and many, many other things. And just like cars, they have to come from somewhere: they have to be manufactured.
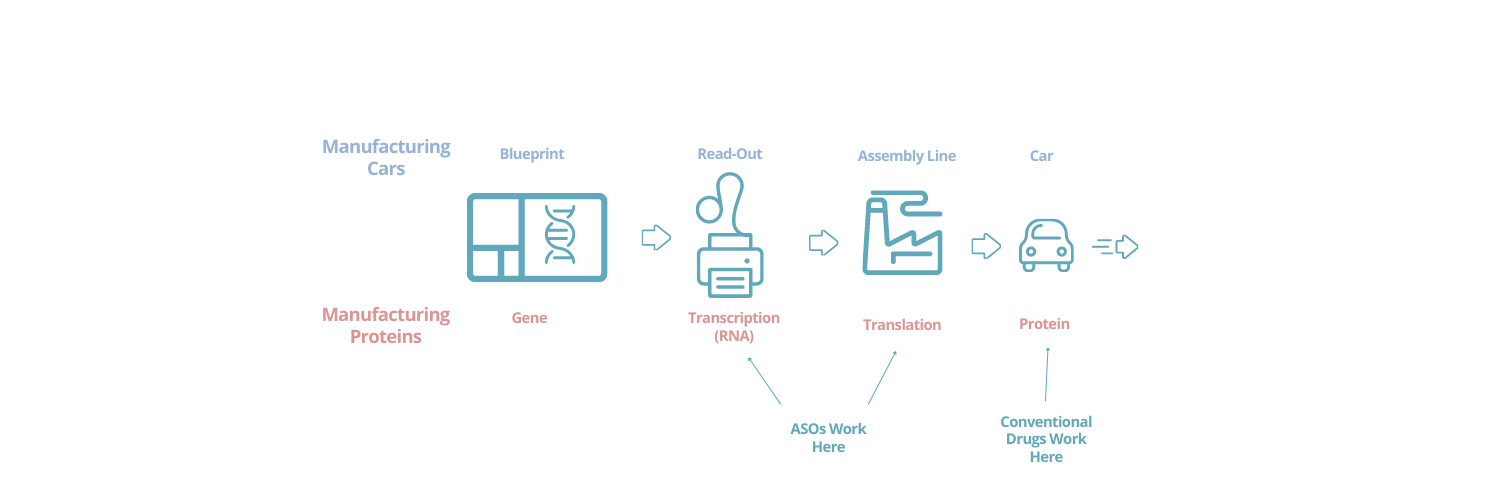
Cars are built based on blueprints. The blueprints are read, and the read-out design is used in the assembly line to manufacture the car. Then the car leaves the plant and is driven and used in many different ways, according to need.
 With proteins, the blueprint is the gene (which is encoded in DNA). The blueprint is read in a process called transcription so that it can be used to make the protein (this creates a complementary molecule called RNA). And then this read-out blueprint is used by the cellular factory in a process called translation to manufacture the protein. Then the protein goes off and can do many things depending on the cues and feedback it encounters in the cell. In a sense, like a car, it can be “driven” in lots of different ways.
With proteins, the blueprint is the gene (which is encoded in DNA). The blueprint is read in a process called transcription so that it can be used to make the protein (this creates a complementary molecule called RNA). And then this read-out blueprint is used by the cellular factory in a process called translation to manufacture the protein. Then the protein goes off and can do many things depending on the cues and feedback it encounters in the cell. In a sense, like a car, it can be “driven” in lots of different ways.
One of the things that makes ASOs so interesting is that, in some circumstances, they can modify the middle steps in this process. That is, the steps where the gene is being read and then converted into the protein. It’s as if you could go into your car manufacturing plant and, without changing the blueprints, add modifications to all of the cars (or only some of the cars, depending on their characteristics). You could change out the wheels or change the engine out (or hang fuzzy dice on the rear-view mirrors!). This would create updated cars, with different capabilities when they are driven. You also could also use this capability to correct manufacturing mistakes. Perhaps there is a weakness in the blueprint that leads some of the cars to be mis-manufactured, but by intervening in the manufacturing process, you can catch and fix this when it happens. Finally, ASOs can sometimes be used to decrease or increase the amount of protein created from a gene (i.e. they can provide fewer or more cars out of the plant).
These capabilities of ASOs are starkly different from the way most conventional drugs work. The vast majority of conventional drugs are believed to work by controlling the function of one or more protein types in cells. In our car analogy, they work entirely by controlling how some of the cars are driven. They can slow them down, speed them up, or make them follow the roads differently in a range of ways. And for many common disorders, this often can work quite well!1

But the landscape is different in many rare disorders, like KS. Often, in common disorders, the underlying drivers of the condition are complex and likely involve many genes, plus environmental factors2. But in rare disorders, there is often a single cause: a single gene, and thanks to genomic sequencing, that gene is known3. So, we have a single gene, that creates a single protein, and there is an issue with it4. Sometimes the cause is that there is too much protein. Sometimes there is too little of the protein, or none at all. And sometimes the protein is being produced in a way that is harmful (think: a car with no brakes) or non-functional (think: a car with no engine). In most cases this traces back to a problem with the gene (i.e. the blueprint). Either the gene is missing (deletion) or damaged in some way (mutation). In our car analogy, you might be able to address some of these issues by changing the way the car is driven (like trying to find a way to slow down the car that lacks brakes), but wouldn’t it be better to simply produce the right car (and the right number of them)?
That, in a nutshell, is why so much work is being done around ASOs for rare disease. They can be particularly helpful in circumstances where there is a single gene causing the disorder, and you know what the issue is with that gene. Of course, they are not a “magic wand”. Developing ASOs as drugs takes lots of work, and there is no guarantee of success. But they open a path that was previously closed: they make it possible to actually go right at the underlying genetic cause of a disorder.
In my next article, I’ll delve a little more into how ASOs work and how, just maybe, we may see an ASO developed for KS in the future.
Until next time,
Eric Scheeff, PhD
IDefine Chief Scientific Officer
1See: aspirin, antibiotics, statins, anti-depressants, etc. The list is quite long…
2A good example of this is Alzheimer’s disease. Experts of are still having good-faith debates over what exactly causes it, even as new drugs are entering the clinic to treat it.
3The official molecular biology term for this is “monogenic disorder” (mono=one, genic=gene).
4Technically, one gene can make multiple versions of a protein as part of its normal function, and often will. But a single protein is a reasonable approximation for our discussion here.

